
Nano Horizons Lab
Zn ion battery
In recent years, the increasing demand for energy and the worsening environmental issues have driven a transformation in power generation. We are now moving towards the use of renewable energy sources such as wind, tidal, and solar power to achieve a safe, reliable, and sustainable energy supply. However, renewable energy sources are mostly unstable and intermittent. For instance, solar power can significantly affect the energy system on cloudy or rainy days and cannot be collected at night. Tidal energy is influenced by the positions of the sun and the moon, leading to significant fluctuations.
To effectively utilize these renewable energy sources and meet the power demands during peak periods, the development of energy storage systems (ESSs) has become urgently important. The development of these storage systems largely depends on highly efficient electrochemical energy technologies. To address today's environmental challenges, these energy technologies must also be safe, cost-effective, and environmentally friendly.
Lithium-ion batteries have dominated the industry for the past 30 years due to their higher energy density (about 250 Wh/kg), lighter weight, and longer lifespan. However, concerns about the flammability and reactivity of organic electrolytes have raised serious safety issues under overcharging or short-circuit conditions. Additionally, the materials (organic lithium salts and organic electrolytes) and manufacturing processes for lithium-ion batteries result in relatively high costs. These limitations have spurred efforts to develop alternative, cost-effective battery technologies.
These factors have collectively driven the development of new battery systems that must prioritize safety, affordability, and low environmental impact. As a result, aqueous rechargeable batteries that combine these three advantages have become a popular research area in recent years. ARBs offer the following advantages:
-
Safety: They use non-flammable aqueous electrolytes instead of flammable organic electrolytes, enhancing battery safety.
-
High Ionic Conductivity: Aqueous electrolytes typically exhibit higher ionic conductivity (usually in the range of 0.1-6 S/cm) compared to non-aqueous electrolytes (usually in the range of 0.001-0.01 S/cm), ensuring rapid charge and discharge and high cycling efficiency.
-
Lower Cost: The electrolyte salts and solvents used in aqueous batteries are more cost-effective, reducing production costs.
-
Simplified Assembly: Aqueous batteries have less stringent assembly requirements since their electrolytes are stable in ambient conditions, eliminating the need for assembly in gloveboxes.
-
Lower Environmental Impact: They have a lower environmental impact compared to many other battery technologies.
In summary, AZIBs hold great promise as a cost-effective, safe, and high-capacity energy storage solution, with the potential to significantly reduce manufacturing costs compared to commonly used lithium-ion batteries.
Anode:
The primary challenge currently facing the anode side is the growth of dendrites. During the charging and discharging processes, electrodes unavoidably generate dendrites. As the number of cycles increases, dendrites continue to grow, eventually piercing the separator membrane and causing a short circuit. The predominant approach in current research to address this issue is to apply a surface barrier layer on the zinc anode's surface to suppress the occurrence of side reactions. In our research, we utilize MOFs (Metal Organic Frameworks) as an anode barrier layer to achieve a dendrite-free anode. MOFs are versatile compounds formed by coordinating metal ions or clusters with organic ligands. Among MOFs, Zeolitic imidazolate frameworks (ZIFs) stand out for various applications. Our study developed a core–shell structure of ZIF-8@ZIF-67 as a protective layer on the Zn anode. To improve ZIF's poor conductivity and Zn affinity, the core–shell structure was hybridized with zincophilic Te, increasing surface area and reducing charge-transfer resistance. Compared to bare Zn, Te-hybridized core–shell Zn exhibited significantly improved cycling performance due to lower nucleation energy and interface resistance.

(a) Schematic of synthesizing Te-hybridized ZIF-8@ZIF-67/NC. SEM image of the morphology of as-prepared. (b) TEM image of a single Te-hybridized ZIF-8@ZIF-67 /NC polyhedron.
Current collector:
Copper metal is commonly used as a current collector due to its excellent conductivity and zinc affinity. However, copper metal current collectors have some drawbacks, such as low Coulombic efficiency and uneven zinc deposition. In our study, we synthesized standing δ-Cu2Te flakes (δ-CTFs) through post-tellurization of Cu foil. The morphology of δ-CTFs was sensitive to growth parameters like temperature and pressure, suggesting a limited range for growing these standing flakes. By adjusting the flux of zinc ions and the electrical field, the lifespan or cycle life has dramatically improved. Finally, we demonstrated a full cell by coupling them with MnO2 as the cathode, showing excellent rate dependence and long-term stability with 1000 cycles while maintaining 100 mAh/g and 100% Coulombic efficiency.

Electrodeposition of Zn on δ-Cu2Te flakes. (a1) The standing δ-Cu2Te flakes served as the current collector. (a2) SEM images of Zn electrodeposited on the δ-Cu2Te flakes. (a3) Magnified SEM images of few flakes, proving the Zn was laterally deposited. Results of Contact angle measurement for (b1) Cu foil, (b2) δ-Cu2Te and (b3) Zn@δ-Cu2Te, respectively.
Cathode:
Currently, extensive research focuses on manganese-based oxides and vanadium-based oxides as cathodes in AZIBs due to their high theoretical capacity (589 mA h/g) and layered structure with enhanced spacing for efficient Zn2+ ion migration. However, manganese oxides tend to dissolve in the electrolyte, leading to reduced cycling lifespans due to structural collapse, while vanadium oxides suffer from a low intrinsic ion diffusion coefficient, resulting in rapid performance degradation. Moreover, the poor electron conductivity in oxide-based materials hampers the reaction kinetics of Zn2+ ions.
In our study, we introduced a novel approach by synthesizing VTe2 through the hydrothermal method for the first time. We systematically explored the impact of pH values on morphology, ranging from nanorods to nanosheets. Additionally, we utilized VTe2 as a cathode in AZIBs, marking a pioneering demonstration. It exhibited a high discharge capacity of 200 mA h/g at 0.2 A/g and maintained 150 mA h/g over 400 cycles at 1 A/g.
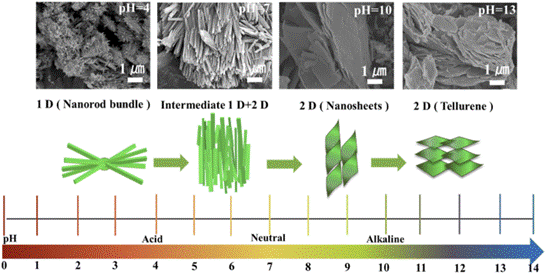
Morphology evolution of VTe2 from 1D nanorods to 2D nanosheets synthesized at different pH values.